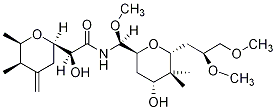
In the late 70s and early 80s, I became interested in chemical communication, and especially in defensive uses of chemical compounds by insects. That included work on a synthetic route to the compound Pederin (a highly irritating material secreted by a rove beetle) supported by a small grant from the Research Corporation. The structure of the target compound is

We proposed pathways to the two substituted tetrahydropyran rings, and a method using imidate intermediates that we expected would serve to join the two halves. Though this project never made it to completion, one of the reactions we proposed for the final step was, in fact, used for that purpose by M. Yanagiya, et al., in a successful total synthesis published in 1979.
Several students worked on approaches to the left-hand side of the compound (as it is drawn above), resulting in one short paper(1). A number of others worked on model approaches to the final steps. Some of that work I did in Professor Jerrold Meinwald’s laboratory at Cornell University, during a 1980-81 sabbatical. During that year I also worked on several other projects, one of which led to the identification of 3(alpha)-hydroxypregn-5-en-20-one (among other steroids) in a defensive secretion of the aquatic bug Abedus herberti.(2) This is one of only a few instances of alpha stereochemistry at C-3 in naturally occurring steroids of this family.
We proposed and tested (3) two routes to the functionality in the central portion of Pederin, which can be thought of as an acylated aminal. Both routes involved imidates – one was acylation of an imidate to yeld an imine that could be reduced to the desired state, and the other was the reaction of an imidate with an alpha-chloroether, to yield a compound that could be converted (by a nucleophilic substitution reaction using pyridinium chloride in DMSO) to the same product (see reactions below). We also carried out some kinetic studies of the acid hydrolysis of various aromatic examples of the acylated aminal structure (4).
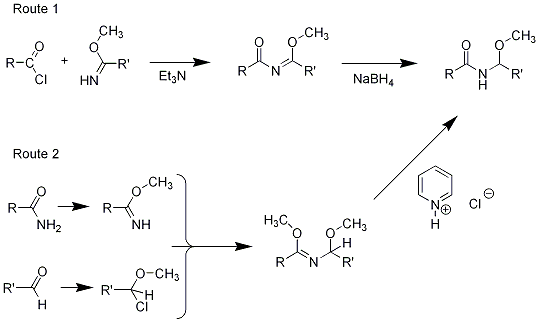
Finally, there was a very interesting observation that we followed up to some extent, but could not make quite reproducible enough to publish. The chloroether used in “Route 2” above could be prepared by the known reaction of an acyl chloride with a methyl acetal in a solvent such as chloroform. We found that, for example, the reaction of acetyl chloride with the dimethylacetal of cyclohexancarboxaldehyde went smoothly, and could be followed easily by nmr spectroscopy. The acetal methyl group peaks decreased while the chloro-ether peaks grew, over a period of an hour or two.
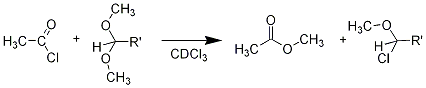
On the other hand, with the acetal of benzaldehyde, and even more notably with that of p-tolualdehyde or other electron-rich aromatic aldehydes, the reaction went somewhat faster, but there was a second, very fast, process, in which the methyl groups of any unreacted acetal starting material and the chloroether product were equilibrating on the nmr time scale. As a result, the process could be followed by watching the methyl ester peak grow, but as soon as the reaction had begun to form products, the O-methyl groups of the acetal and chloroether collapsed into a single broadened peak that slowly shifted from a chemical shift near that of the pure acetal to one near that of the pure product. Eventually, only product was present and the spectrum became clearly that of methyl acetate plus the product chloroether, with only sharp lines. We postulated a process something like the following, and carried out a number of experiments to explore the reactions, but it was difficult to obtain completely pure starting materials, so that traces of acid or other possible catalysts prevented our sorting out the relative rates, etc.
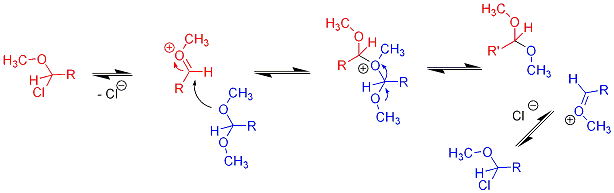
Over time the starting acetal was entirely converted to the chloroether, but as long as both were present in the reaction mixture this exchange was going on.
(1) Preparation of 2-Oxazolines from Lactones, with P. B. Comita* and K. M.
Rowland, Jr.*, J. Org. Chem., 1977, 42, 1467.
(2) Pregnanes from Defensive Glands of a Belostomatid Bug, with R. Smith, T.
Eisner, and J Meinwald, Experientia, 1993, 49, 175-176.
(3) Synthesis of N-(alpha-Methoxyalkyl)-amides from Imidates, with J. Fischer*,
W. Bartz*, and J. Meinwald, J. Org. Chem., 1985, 50, 5609.
(4) Substituent Effects on the Hydrolysis Rate of N-(alpha-methoxybenzyl)
Benzamide, with Janie B. Schneider* and Jennifer L. Herek*, Abstracts of Papers,
32nd
National Organic Symposium, June 1991, Minneapolis, MN (Poster)